Republican Attacks Against Vaccines Put Our Kid's COVID Vaccine Availability at Risk. Call The FDA and Demand They Approve Novavax as an mRNA Alternative for Children.
Food and Drug Administration (FDA)
Don't let Republican attacks on vaccines leave our kids unprotected.
We have to not only protect our vaccines but also improve on them.
Call to action: There is substantial risk that Republicans could remove access to mRNA altogether, and that would mean we would have no COVID vaccine for under twelve.
We need the FDA to use the Emergency Use Authorization that Novavax is still under and approve their vaccine for pediatric access as soon as possible.
We, the public, demand that you use the Emergency Use Authorization to expand Novavax access to include pediatric use.
Novavax for under 12 is the same vaccine used for every other age group.
And all other COVID vaccines for under 12 are still under EUA.
We need the FDA to immediately use this process to expand access for all age groups.
There are current arrangements to seek approval of pediatric expansion much later this year, but that's all based on a deal made by the Biden administration, and it's unclear if it will be honored.
Instead, the FDA should fast-track approval and make it available as fast as possible.
We both ask that you sign the petition and also contact various people throughout the FDA to show them there is public support for this.
Because this is a big ask, there is a substantial supplementary document to explain all the details of why this is possible and how it can be done.
That can be found here ---> https://tinyurl.com/5n7en4j4
But we need to act right away...
Because there are some very concerning things being said…
Trump’s pick for HHS, Robert Kennedy Jr., has expressed some pretty clear positions on the issue.
And it doesn’t stop there…
Comments from the writers of the Great Barrington Declaration are even more unsettling…
A signing member, Jay Bhattacharya, has been nominated to lead the NIH.
Here’s an example of his opinions…
Unethical infection-based immunity could be the only option for our kids.
Here, see for yourself…
It’s not great.
The only silver lining is that most of this negativity was framed in the past context of mRNA mandates.
Well, until you get to Florida.
This is an actual screenshot from the official Florida public health…
There are many things wrong with the above statement.
I don’t even know where to start, to be honest…
That being said…
Florida Surgeon General Joseph Ladapo is possibly up for a major health position.
And he tweeted this on November 27th, 2024…
Trump’s former CDC Director, Dr. Robert Redfield, said in August 2024 that he prefers Novavax.
While RFK himself has made similar statements.
Though I admit it was a few years ago.
But why Novavax?
It comes down to four main ideas...
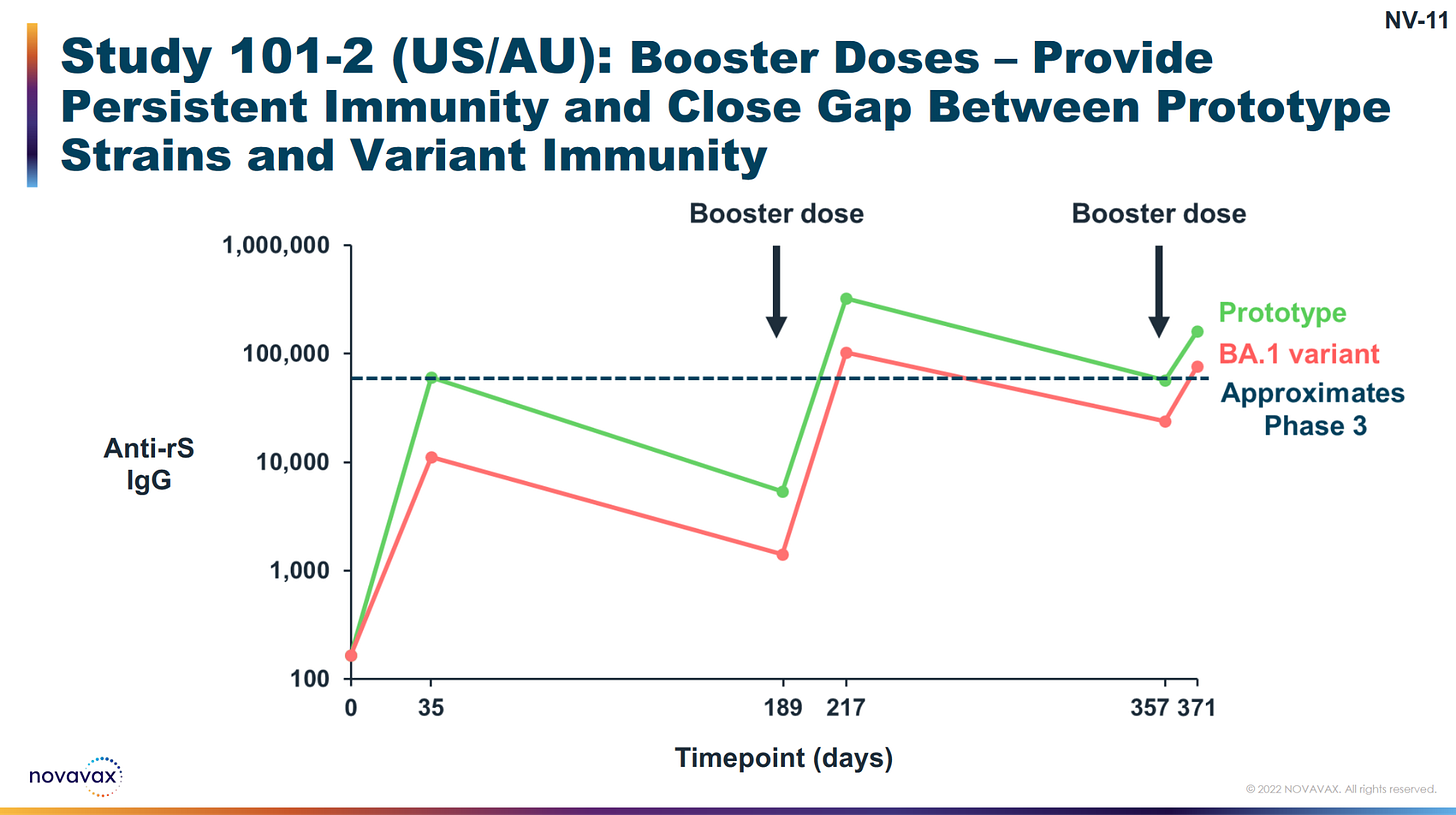
1) Year-round protection without gaps (persistent immunity).
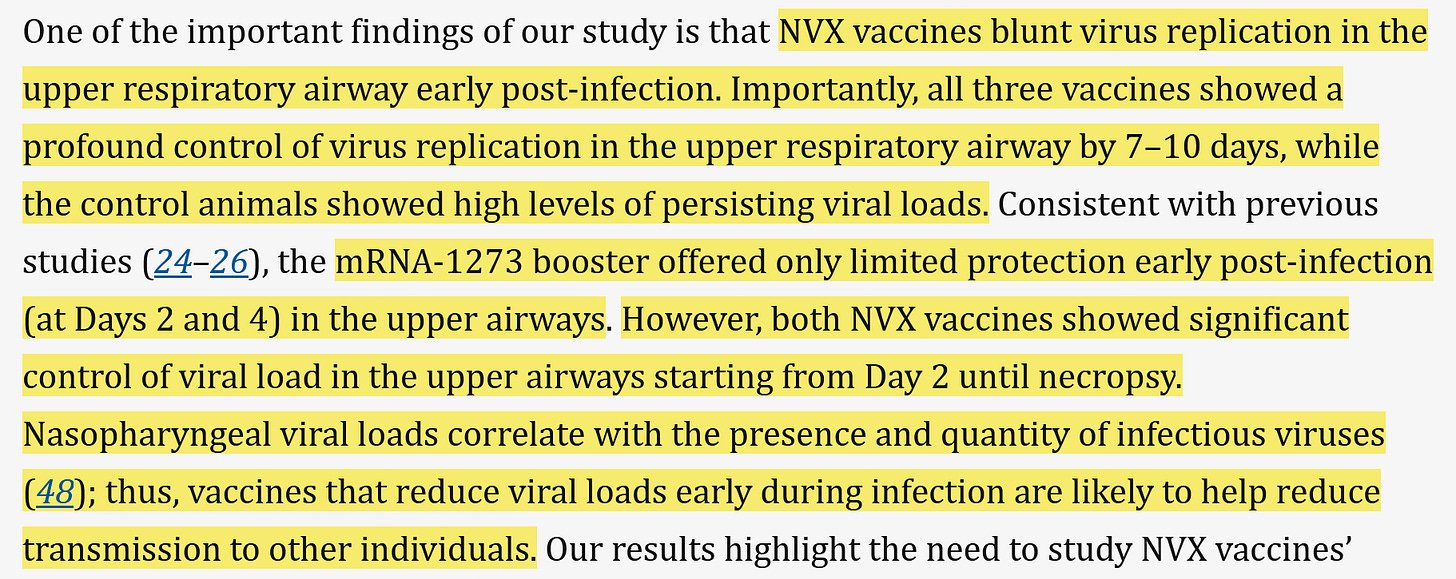
2) A more effective upper respiratory tract response.
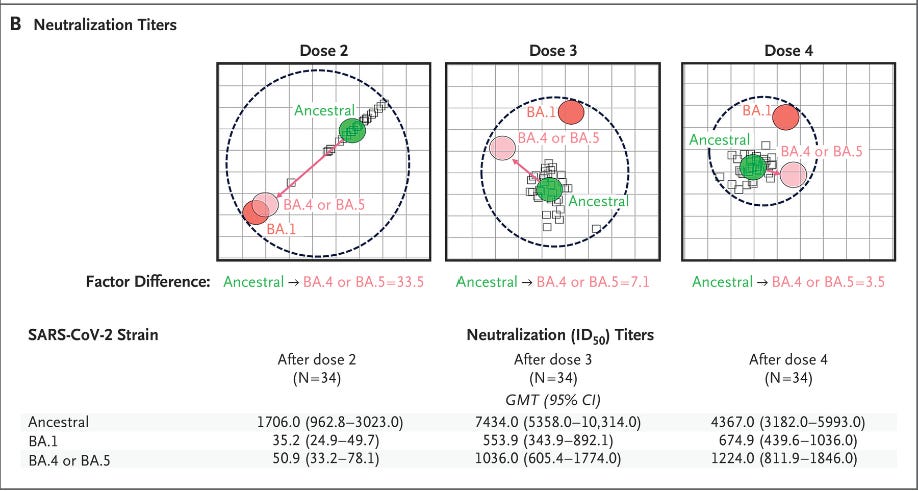
3) Increasing the breadth of your antibodies with additional shots.
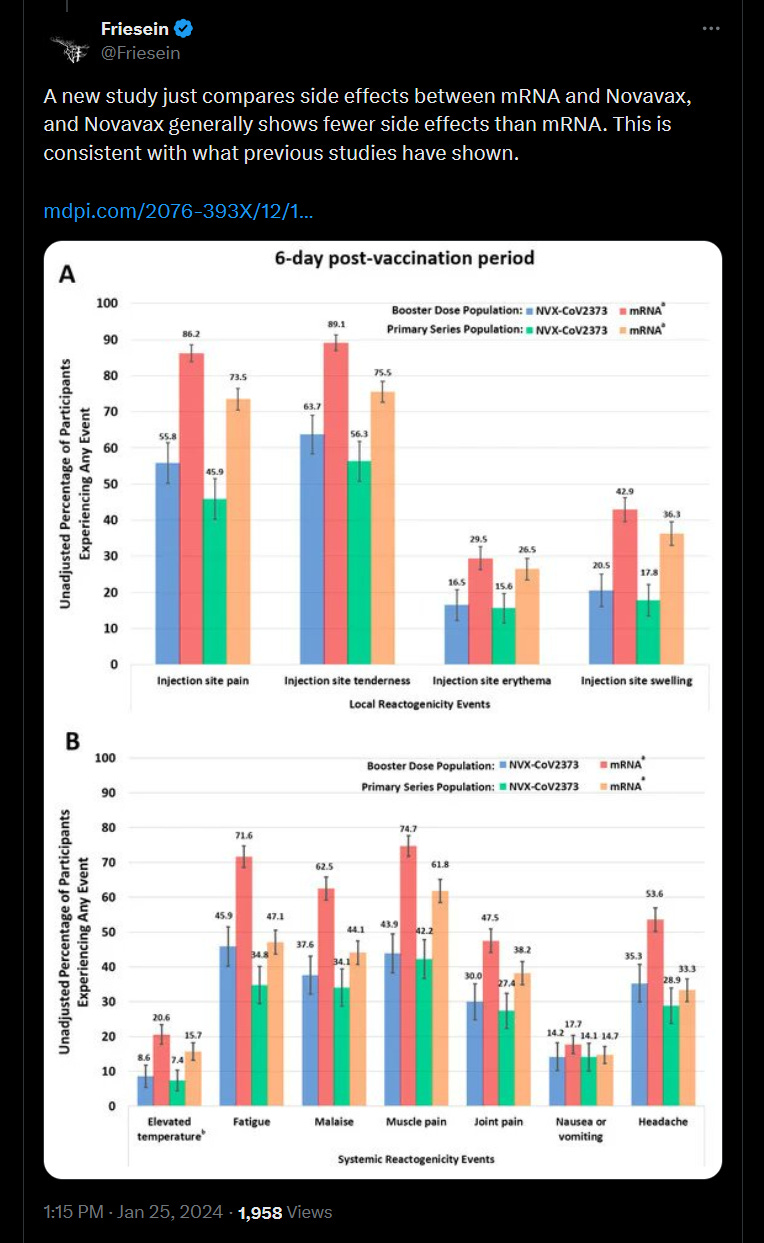
4) Fewer adverse events.
The FDA is demanding outdated testing requirements.
We've already been pushing on the FDA to change their testing parameters...

Getting the FDA to approve Novavax for kids before the FDA flips to Republican control is critical if we want to have pediatric COVID vaccines approved next year.
There is reasonable concern that they will not recommend mRNA anymore, and it's unlikely they will approve new pediatric vaccines in the first year...
That could leave us with no way to vaccinate our children.
We should get ahead of that risk and push the FDA to approve Novavax access for kids right now.
There are many reasons to have a protein-based COVID vaccine available...
And this is not the approval of a new vaccine; this is expanding existing access to the exact same vaccine.
The most protective COVID vaccine has just been sitting on the shelves while our kids get infected over and over again.
Pediatric Novavax has been proposed and tested as the exact same vaccine that we already have, unlike the overly complicated dosages of mRNA...
It’s all the same shot.
This is from the adult vaccine trials.
And this is from the pediatric trials.
The FDA could snap their fingers, and it could be almost instantly available.
And it all comes down to the fingers of Peter Marks snapping. He's the head of CBER and was the focus of our previous successful action to get COVID vaccines released earlier this year.

What's going on is that Novavax is being kept on EUA. Though they can, as of last year, advertise their product, there are still a lot of limitations set by the FDA.
They are applying for full approval, but the FDA is making it more challenging for them than the other vaccine manufacturers, which they have been doing the whole time.
The EUA gives the FDA tons and tons of power over what Novavax can do or say...
However, the EUA also allows for pediatric access to be approved faster via emergency authorization.
Then why aren't we doing that?
The problem is that Novavax has made an arrangement with the FDA where they will only ask for pediatric Novavax expansion once their full approval has gone through. This process is called the Biologics License Application (BLA), noted in the SEC filing, and they would file pediatric expansion as a supplement to that approval. This process is being held up year after year for no clear reason.
This would be the wrong way for the FDA to handle this situation unless they DID NOT want the product to come to market. And even though Americans paid for Novavax via Operation Warp Speed, other countries are getting ahead of us on pediatric access.
Japan has approved ages 6 - 12 in just the last few months.
The adjuvant has been used in multiple vaccines, including the R/21 Oxford vaccine, which increased real-world effectiveness significantly. While this vaccine was intended for children six months and older.
Call the FDA and demand they approve Novavax expansion for pediatric use.
And don't let them fool you; the ball is entirely in their court.
We have many contacts you can try.
We need to start now and get ready for a real push with the new administration.
This will not only keep up the fight to protect our vaccines, but it will also make it clear to the new FDA that there is demand for Novavax and especially for pediatric expansion...
That way, when they apply for their general approval, we can ensure it goes through.
Until then, while the vaccine is under EUA, the FDA can approve pediatric expansion right away, and the product we would use is already sitting on the shelves, just letting its three-month expiration date tick down.
The "Ball" is in the FDA's court, according to Novavax's SEC filings.
So, sign the petition and let's start making contact with the FDA... While it's still there, at least.
This list will be updated as needed for any new FDA members of concern.
We have a few different contacts to focus on here...
General Contact: 1-800-835-4709
Director of CBER - Peter Marks, MD, PhD
204-402-8116
240-402-8000
Peter.Marks@fda.hhs.gov
This is one of the main people holding us up. Reminder: He also held up Lucira, which helped Pfizer buy the company after it went bankrupt due to a lack of approval.
CBER stands for Center for Biologics Evaluation and Research; they are responsible for approval.
OVRR, DIRECTOR - Office of Vaccines Research and Review
OFFICE DIRECTOR - David Kaslow, MD
Organization: DHHS/FDA/CBER/CBER/OVRR
301-796-7114
David.Kaslow@fda.hhs.gov
OVRR, Associate Director for Research - Tod Merkel, PhD
Organization: DHHS/FDA/CBER/CBER/OVRR
240-402-9746
Tod.Merkel@fda.hhs.gov
OVRR, Regulatory Review Branch 1
SUPERVISORY BIOLOGIST - Jon Daugherty, PhD
Organization: DHHS/FDA/CBER/CBER/OVRR/DRMRR/RRB1
301-796-2640
Jon.Daugherty@fda.hhs.gov
OVRR, Regulatory Review Branch 2
SUPERVISORY BIOLOGIST - Rakesh Pandey, PhD
Organization: DHHS/FDA/CBER/CBER/OVRR/DRMRR/RRB2
301-796-2640
Rakesh.Pandey@fda.hhs.gov
OVRR, Regulatory Review Branch 3
SUPERVISORY CHEMIST - Elizabeth M. Sutkowski, PhD
Organization: DHHS/FDA/CBER/CBER/OVRR/DRMRR/RRB3
301-796-1536
E-mail Elizabeth.Sutkowski@fda.hhs.gov
OVRR, Clinical Review Branch 1
SENIOR ADVISOR FOR THERAPEUTICS - Maria Allende, MD
Organization: DHHS/FDA/CBER/CBER/OVRR/DCTR
301-796-2952
Maria.Allende@fda.hhs.gov
OVRR, Clinical Review Branch 2
SUPERVISORY PHYSICIAN - Andrea Hulse (James), MD
Organization: DHHS/FDA/CBER/CBER/OVRR/DCTR/CRB2
301-796-2640
Andrea.Hulse@fda.hhs.gov
OVRR, Clinical Review Branch 3
SUPERVISORY PHYSICIAN - Anuja Rastogi, MD, MHS
Organization: DHHS/FDA/CBER/CBER/OVRR/DCTR/CRB3
301-796-1544
Anuja.Rastogi@fda.hhs.gov
OFFICE OF THE CENTER DIRECTOR, Chief, Executive Operations Staff
SUPERVISORY MANAGEMENT OFFICER - Debra Fisher, MBA
Organization: DHHS/FDA/CBER/CBER/OD/EOS
240-402-3003
Debra.Fisher@fda.hhs.gov
OFFICE OF COMPLIANCE AND BIOLOGICS QUALITY
Special Assistant to the Director
CONSUMER SAFETY OFFICER - Mona G. Atkinson, MS, MBA
Organization: DHHS/FDA/CBER/CBER/OCBQ
301-796-4215
Mona.Atkinson@fda.hhs.gov
MANAGEMENT OFFICER - Gloria Grantham
Organization: DHHS/FDA/CBER/CBER/OD/EOS
Gloria.Grantham@fda.hhs.gov
And a few more emails to try:
cberocod@fda.hhs.gov
OCOD@fda.hhs.gov
commissioner@fda.hhs.gov
To:
Food and Drug Administration (FDA)
From:
[Your Name]
Dear FDA,
We, the public, demand you use the Emergency Use Authorization to expand Novavax access to include pediatric use.
Novavax for under 12 is the same vaccine used by every other age group.
And all other COVID vaccines for under 12 are still under EUA.
We need the FDA to immediately use this process to expand access for all age groups.
Thank you.