Success! We pushed for earlier approval and clearer language... We got both! Now we finished the job and Novavax is approved!
Food and Drug Administration (FDA)

Update: Demand The FDA Approve Novavax.
We Did It! Novavax Is Approved.
August 30th Update: Novavax is approved! There is still helpful data below and we will update this space for the next steps in timing and pediatric approval.
August 29th Update: Huge success! As of August 22nd, we were able to push the FDA to have an earlier COVID vaccination season and with that they made clearer guidance on vaccine access.
This petition and the actions that came with it were almost 100% successful.
This is coming along with a few other items we've been pushing for as a group...
But we're not quite across the finish line.
It's looking like we will see another vaccine push in the spring to protect from a potential 2025 Summer Wave. It could be as soon as late winter. That means folks will likely be getting two updated vaccines this year which will also update their memory response... A long time demand from our group!
This could be either a shot in the middle of winter or a shot in the spring but it signifies a major change in our COVID response as the pendulum swings away from treating COVID like the flu and toward treating it as it’s own unique problem.
But this excitement also comes with a big caveat...
Even though Novavax filed before any other company, their approval is being delayed AGAIN.
That means we have to continue contacting the FDA.
We have some new contacts to focus on here...
General Contact: 1-800-835-4709
Director of CBER - Peter Marks: <-- this is one of the main people holding us up. Reminder: He also held up Lucira, which helped Pfizer buy the company after they went bankrupt due to lack of approval.
Phone: 204-402-8116
Email: peter.marks@fda.hhs.gov
CBER stands for Center for Biologics Evaluation and Research; they are responsible for approval.
Director, Office of Vaccines Research and Review, CBER, FDA - David Kaslow
Phone: 301-796-2640
Email: david.kaslow@fda.hhs.gov
Special Assistant to the Director - Mona G. Atkinson, MS, MBA: 301-796-4215
Regulatory Review - Jon Daugherty, PhD:
301-796-2640
Associate Research Director - Tod Merkel: 240-402-9746
Rakesh Pandey, PhD
301-796-2640
Elizabeth M. Sutkowski, PhD
301-796-1536
Maria Allende, MD
301-796-2952
Andrea Hulse (James), MD
301-796-2640
Anuja Rastogi, MD, MHS
301-796-1544
And a few more emails to try:
commissioner@fda.hhs.gov
debra.fisher@fda.hhs.gov
Gloria.grantham@fda.hhs.gov
cberocod@fda.hhs.gov
But what's going on here?
Every year Novavax is held back weeks or even months after mRNA, often with restrictions on access. The good news is the two month protocol we've been driving for the last year has now been accepted for all COVID vaccines. Now it's possible anyone will be able to get a second COVID vaccine, "as long as they are two months after their last."
Right now, folks are only approved for a single updated vaccine, but VRBPAC and ACIP are expected to meet in October and this idea will be discussed further. However, the language on that approval has already shifted to as long as it has been two months since your last.
This means that folks should be able to get two Novavax, two months apart without jumping through the hoops of last year just to complete a primary series... At worst, it will be possible to time the winter and spring vaccination process to the back of the winter and the start of spring, but more on that in an actual article.
Let's address the elephant in the room: JN.1 vs KP.2
Is one really more updated than the other?
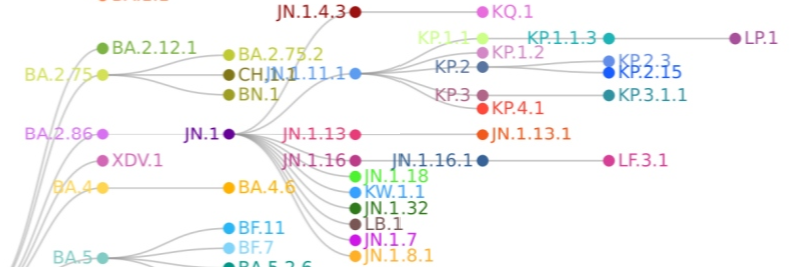
JN.1 is the "trunk of the tree" of the new variant line that started with BA.2.86.
If you rotate it 90 degrees, it actually looks like a tree... BA.2.86 represents the roots, JN.1 is the trunk.
The primary focus of the vaccine is the spike protein.
In this context, the variants are only four mutations apart.
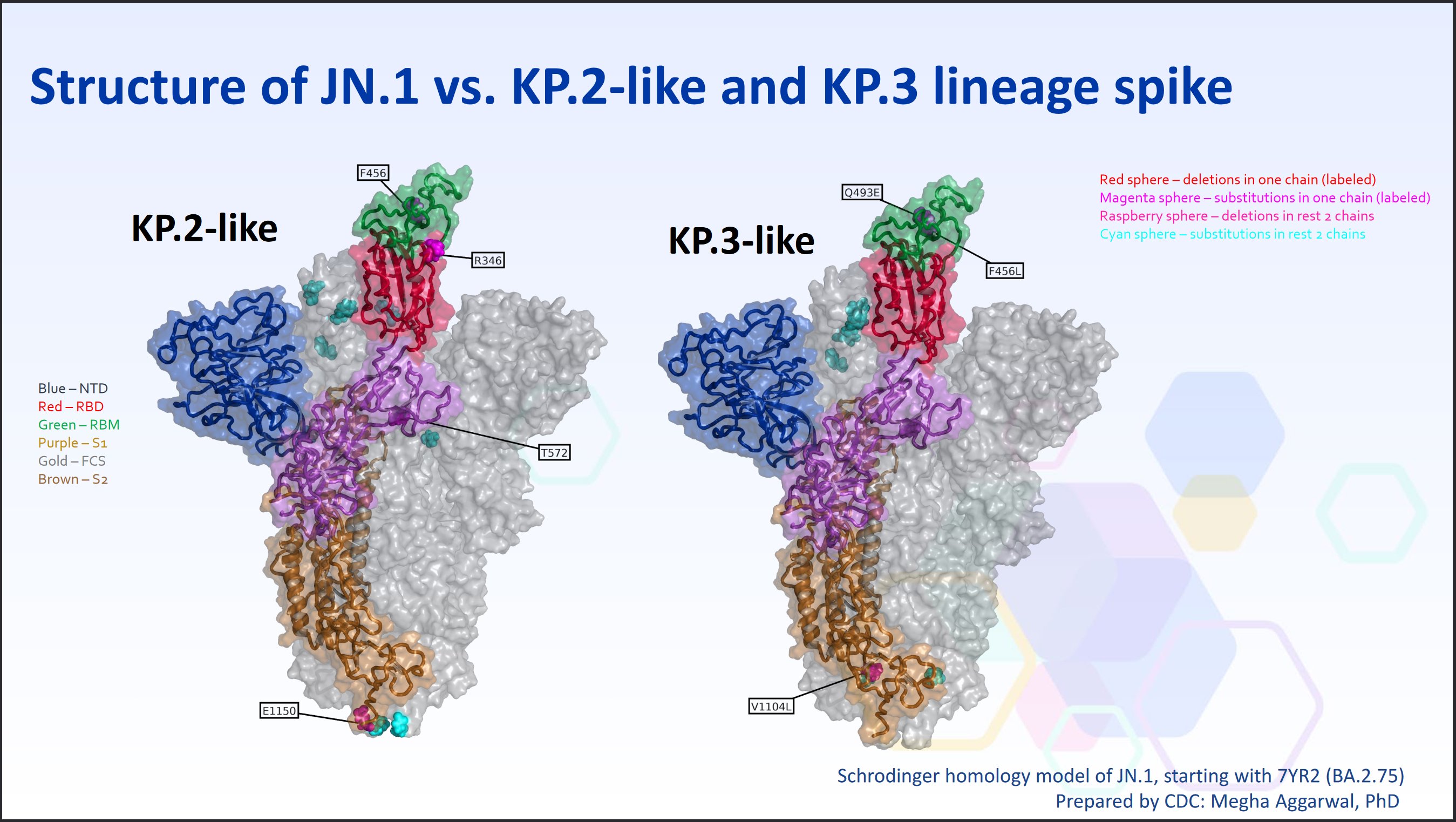
Below is a visual representation from the CDC... The base spike is demonstrated as the JN.1 spike, and the boxes demonstrate mutational differences.
The one on the left is KP.2 which is four mutations different than JN.1, and KP.3 on the right is only three.
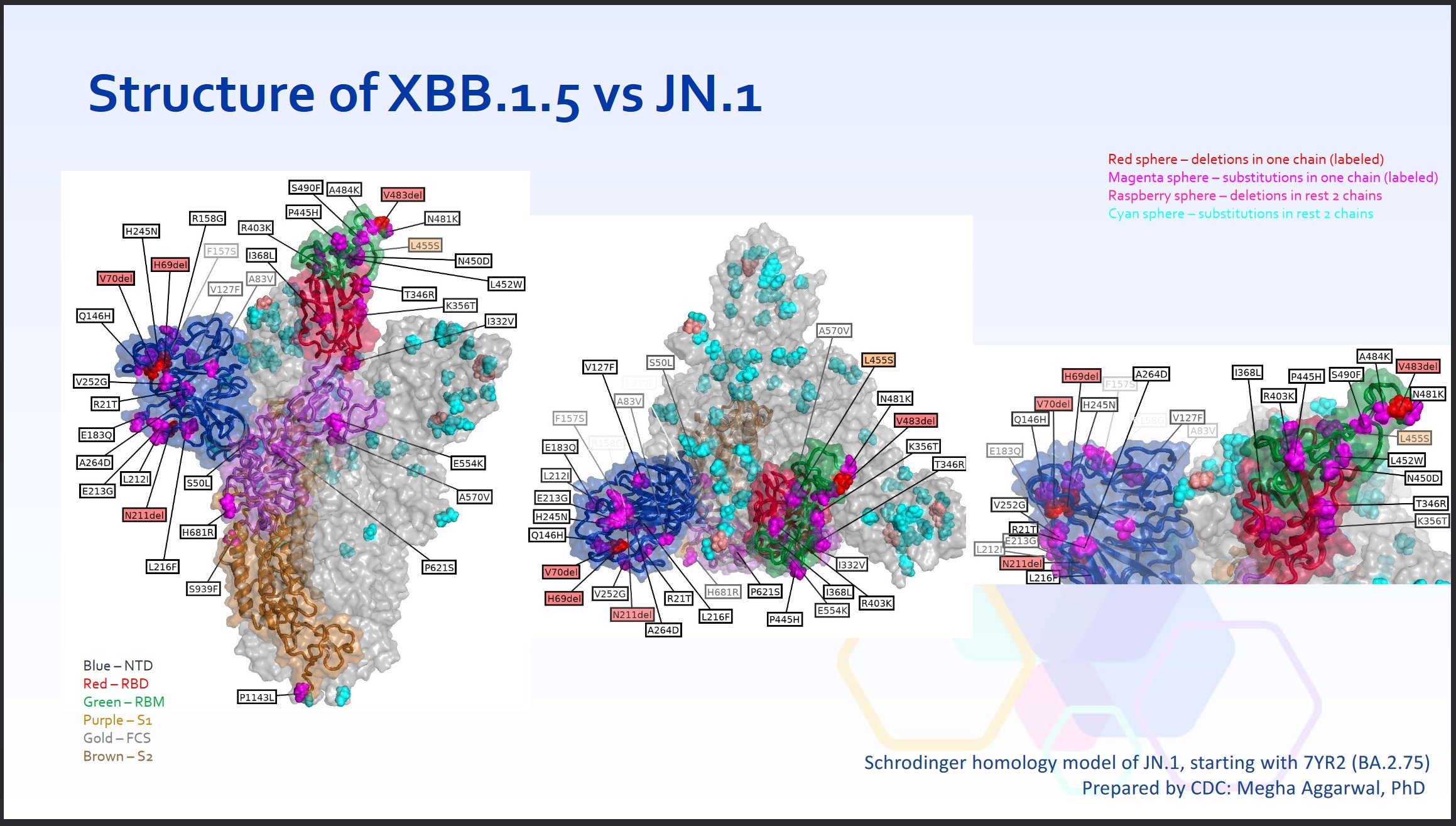
For comparison, the jump from XBB.1.5 to JN.1 was MUCH more significant.
This is a type of jump that requires an update to our memory response.
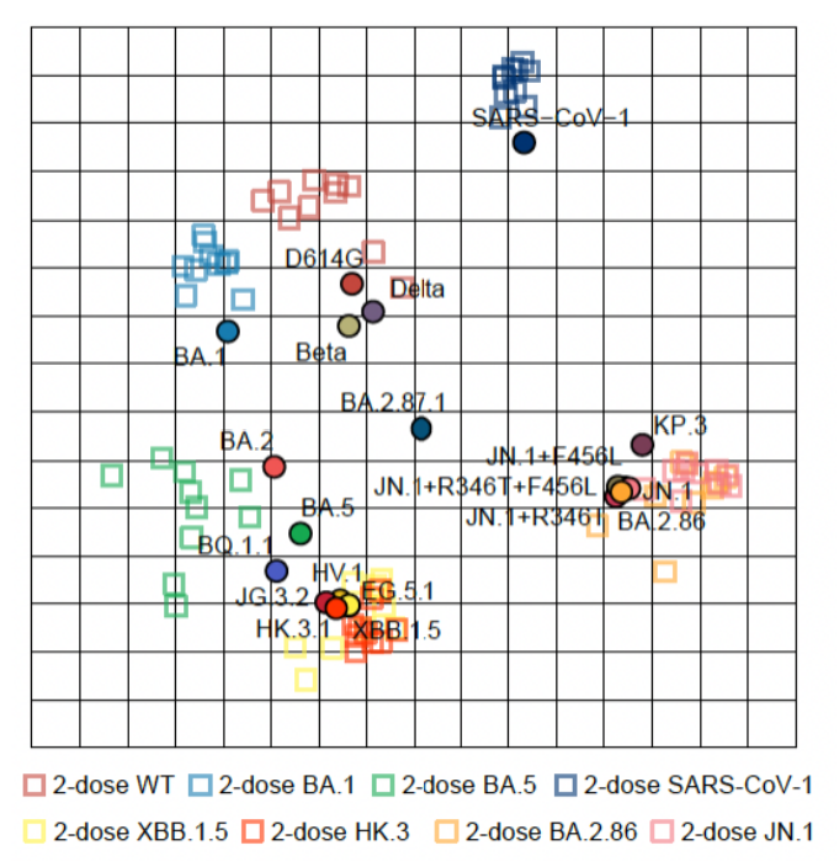
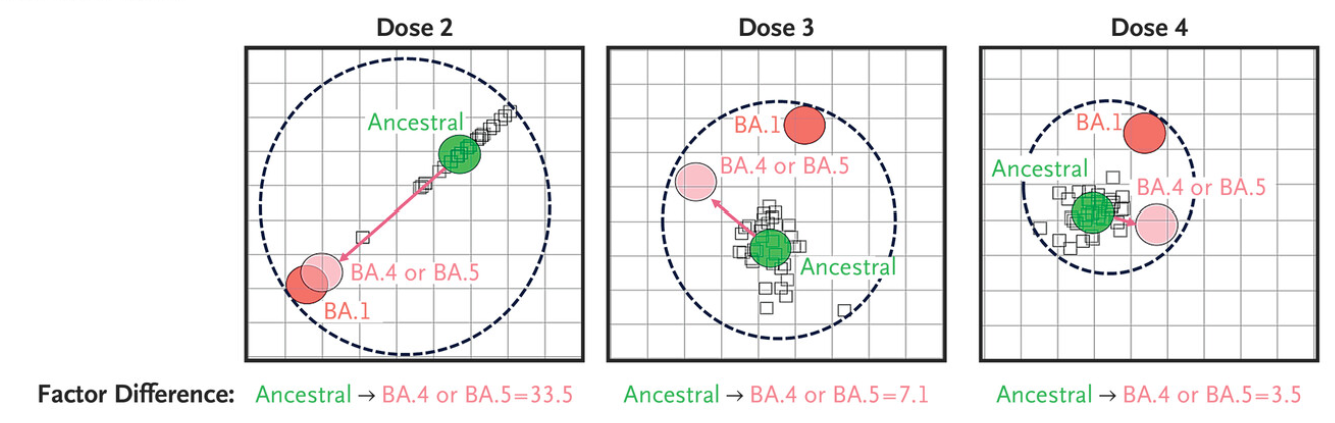
For comparison... This chart below shows the antigenic distance between variants and the distance between antibodies created by two vaccinations of each type.
The jump from SARS1 to BA.1 Omicron was as significant as the jump from XBB.1.5 to JN.1.
Imagine that each square is a square mile and think of the distance like you would a map.
Even how we think of antibodies gained from vaccination isn't quite right because they don't end up exactly literally on top of the variant we are aiming for...
They end up a little to one direction or another but this is as close as you can get on a population level.
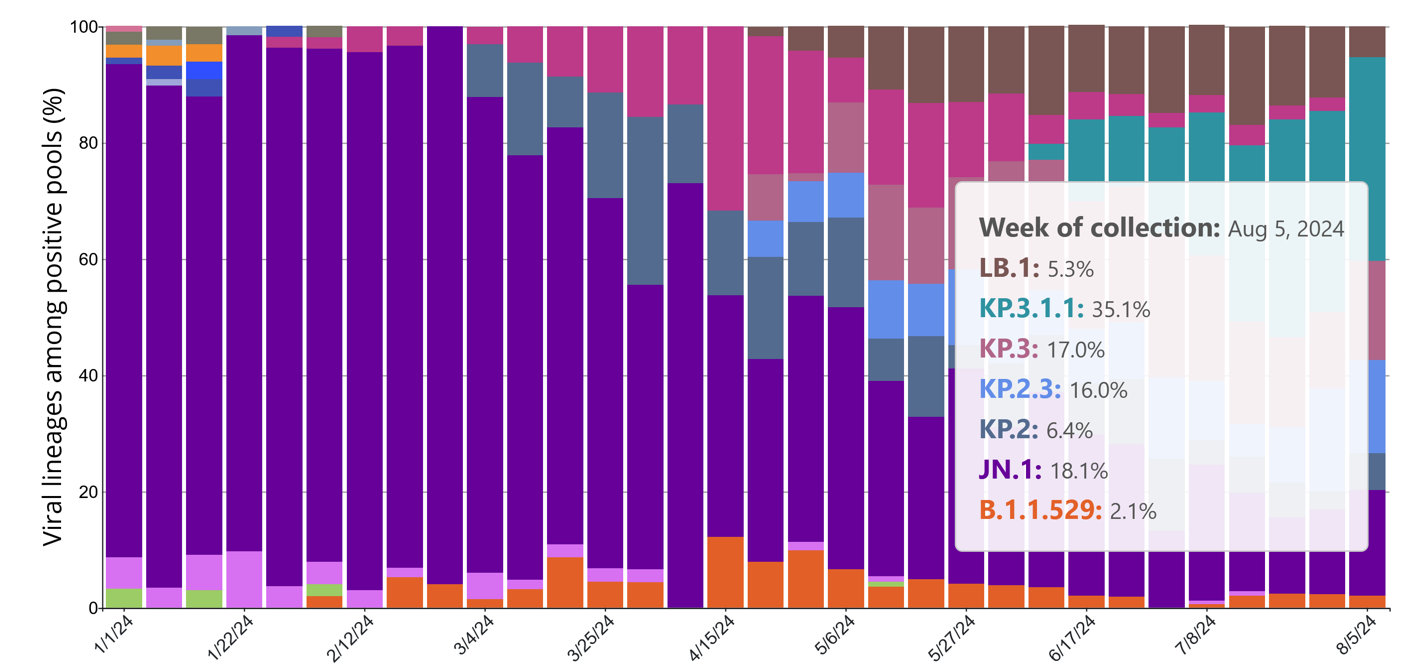
But what about the variants now?
As of Aug 5, 2024’s collection of positive pools, KP.3.1.1 is the dominant variant at 35.1% and JN.1 is next at 18.1%.
Collectively they make up 53.2% of all lineages showing up in positive pools.
Just in case you missed it, that's more than half of all variants.
Let's take a look at the vaccine manufacturers data from the last VRBPAC.
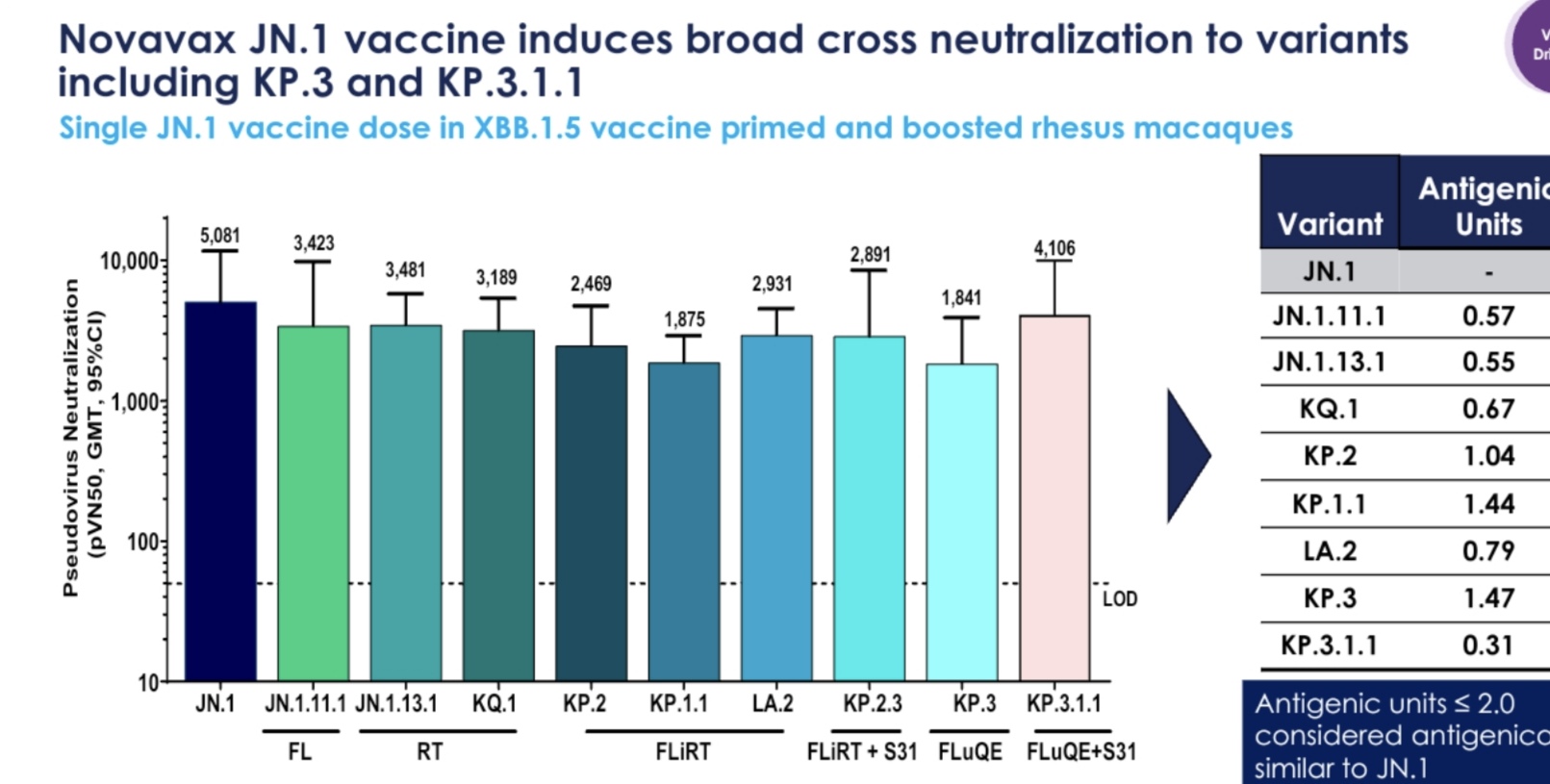
Starting with Novavax.
Their data very clearly demonstrates that JN.1 and KP.3.1.1 have the strongest response.
Meaning that Novavax has the strongest response against 53.2% of current active variants.
Let's have a look at Moderna's data next...
This demonstrates that their KP.2 vaccine had lower efficacy than their JN.1 vaccine.
Both were still very good, but it's clear which was performing better.
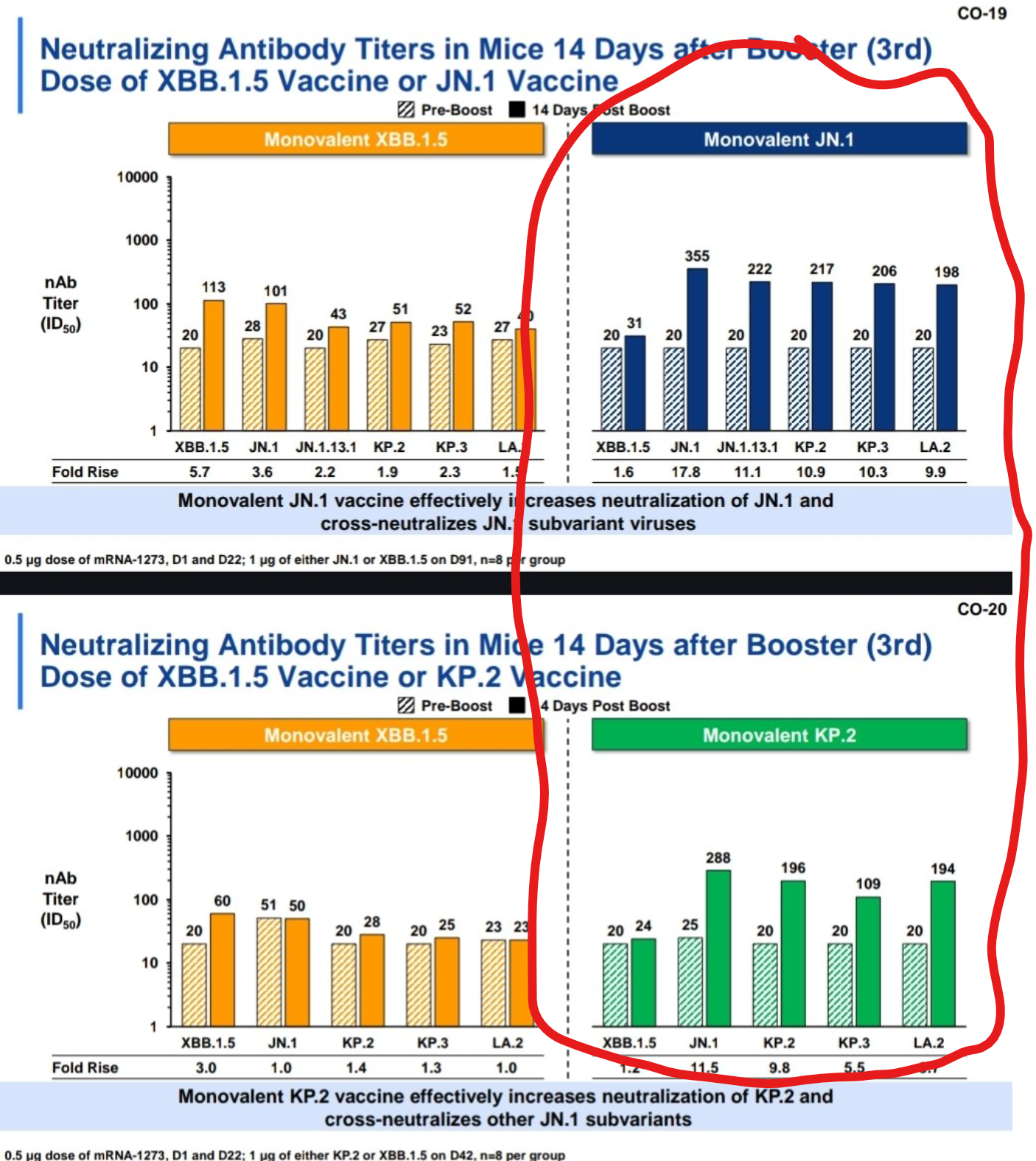
![]() And then Pfizer's data...
And then Pfizer's data...
Pfizer's data is actually a bit different than the other two.
While the other two used an XBB.1.5 first then boosted with a single JN.1 or KP.2 vaccine...
Pfizer has decided to test XBB.1.5, JN.1, and KP.2 all on top of a bivalent of an original crossed with a BA.4/5... and this is a bit unclear, but it makes me think a trivalent was actually used.

So, while this data does show a promising response to KP.3 variants, it's based on boosting with a vaccine that no one has received... so we'd need more standardized modeling to make a claim either way regarding Pfizer's KP.2 vaccine.
This data simply cannot be used in a comparative manner in the same way the other two can.
I hope that helps.
Sign the petition for email updates to be notified when we have a new call to action.
(End of Update)
We are in unprecedented territory...
Normally, COVID vaccine updates would roll out in the fall, but as we are learning more about COVID, and how we can't treat it like the flu, vaccine manufacturers are rising to the occasion. Between advanced mutations that require shifts in memory response, an earlier cold and flu season, the Bridge Access Program ending in August, and schools being discovered as major vectors for infection...
We can't wait for the fall to roll out updated vaccines.
Both Moderna and Novavax have submitted to the FDA for immediate approval of their JN.1 updates that they have said can be delivered between mid-July and early August. KP.2 vaccines won't be ready or available until we are preparing for the winter surge.
Moderna
Novavax
Both updated vaccines need to be approved for release as fast as possible.
The public needs access to updated vaccines before the school year starts.
Earlier rollout means protecting kids and their families.
Earlier rollout means that vaccination with updated vaccines will be possible before school starts, which will reduce transmission for the entire population. Schools will remain a high-risk environment for transmission until proper clean air procedures are implemented, so we should shift our policy to release updated vaccines prior to the school year starting.
This will help put parents at ease when they send their kids off to school.
Cold and Flu season is starting earlier.
The “cold and flu season”, which now includes COVID, starts earlier than it did previously. With the jump from XBB to JN.1 being so significant, immune imprinting is an issue for anyone who is avoiding infection and trying to stay prepared for an unexpected exposure. This means they need a two shot series two months apart regardless of mRNA or protein based vaccine platform.
An earlier rollout would allow people to reset their
memory response in time for a winter surge or we run the risk of having an even worse surge than
our previous winters.
Our outdated memory response is a concern that must be addressed.
There is a way to aim for variants we can’t see yet.
Should people choose Novavax to update their memory response, then it will also help them improve their reaction to new variants. An effect of Novavax’s adjuvant is that after multiple doses, it boosts existing antibodies to challenge new mutations.
We can’t accurately predict which variants are coming next, so the Matrix-M adjuvant allows the JN.1 vaccine to respond better to future variants.
This allows the JN.1 vaccine to create a response
that will reach future variants we can’t see yet.
An earlier release would allow access through the Bridge Access Program.
The Bridge Access Program is ending in August and rolling out the updated vaccines in July-August would help make these vaccines more available to underserved communities who benefit the most from greater vaccine access
The private market has made COVID vaccines unreachable for the uninsured and we need to do everything we can to maintain equitable access to these updated vaccines and future ones.
We can't let access to insurance inhibit access to these lifesaving vaccines.
The public needs both an mRNA and protein-based vaccine option.
The FDA has approved Novavax notoriously late over the last few seasons, but some people can not get mRNA so this has become an equity issue. These people are often immuncompromised and at the highest risk, their protection cannot be delayed.
Both of these vaccines should be approved on the same timeline so that
both mRNA and protein-based vaccines can be available at the same time.
Also, the CDC & FDA should make it easier for people to access two shots in a two month period.
Please call the FDA to demand they do all of the following:
- Urgently approve the JN.1 vaccines for mid-July/early August release, before the school year starts.
- Make vaccine rollouts available in the spring and fall to protect against summer and winter surges.
- Expedite the approval of the pediatric Novavax which will offer a protein-based option for children.
- Make clearer rules so those who want/need additional doses can get them without restrictions.
Call the FDA at 1-888-463-6332 (when prompted, press 3 to speak to representative, then press 1 for vaccines department) and demand all of the above.
Sign this petition to send a message to the FDA and CDC that the public wants the vaccine rollout to happen earlier and that you want want greater access to these vaccines.
To:
Food and Drug Administration (FDA)
From:
[Your Name]
We demand the FDA and CDC approve updated COVID vaccines for an early release and clearer guidance on vaccine access.
Both the updated mRNA and protein-based vaccines need to be made available as fast as possible.
The public needs earlier access to updated COVID vaccines. Not everyone can use mRNA vaccines and that means we need both options available at initial rollout, before the school year starts.
We also need clearer guidance to provide access to additional vaccines for all. It is time to revisit the idea of a two-shot series for immunocompromised AND immunocompetent people, which should accompany an earlier release of the updated vaccine.
Reasons:
- Many people have not gotten vaccinated since their initial vaccination.
- The “cold and flu” season is starting earlier, requiring an update to our policy.
- The significant jump from XBB to JN.1 requires updating our memory responses.
Benefits:
- Kids can get fully vaccinated before the school year starts.
- This would give people time to get a two-shot series before a winter surge.
- An earlier release would allow people access via the Bridge Access Program.
- Earlier access would allow the global south to see an updated vaccine, as well.
Both Moderna and Novavax have submitted to the FDA for immediate approval of their JN.1 updates that they have said can be delivered between mid-July and early August.
Between advanced mutations that require shifts in memory response, an earlier cold and flu season, the Bridge Access Program ending in August, and schools being discovered as major vectors for infection...
The public needs updated COVID vaccines earlier than the planned Fall rollout that we normally associate with the flu. Vaccine manufacturers are prepared to have both mRNA and protein-based options available for a mid-July - early August release.
The flu and cold season is not only starting earlier but hitting harder while at the same time there have been SIGNIFICANT jumps in mutation requiring updating memory responses…
This year we are facing unprecedented challenges with viral mutation and a lack of mitigations. Not only do we need to make both the updated mRNA and protein-based vaccines available as quickly as possible, but we also need to have clear language allowing two shots two months apart so as to not confuse doctors, pharmacists, or patients.
This earlier rollout will not only allow folks to reset their memory response before the winter surge but it will also let kids get vaccinated before the school year starts.
While at the same time, giving folks the opportunity to get their updated vaccine, and should they choose Novavax, then the multiple shots will help their immune response reach future variants.
And while this early release might allow folks access through the Bridge Access Program, it is to the benefit of the entire country that the life of that program be extended for the foreseeable future.